00971504825082
📄 Print PDF
00971504825082
Static Electricity


There are two types of electric charges:
Negative (-) and Positive (+)
What does it mean for an object to be positively charged?
What does it mean for an object to be negatively charged?

Any material consists of
A nucleus containing positively charged protons and neutral neutrons
Electrons with negative charge orbit around the nucleus
Is the atom electrically neutral?
Because the number of protons = number of electrons
Proton charge = Electron charge
\[ 𝑞_𝑒 = 𝑞_𝑝 = 1.602 X 10^{-19}C\]
Electrons are the particles that are gained or lost
Charged objects contain protons and electrons but their numbers differ
A negatively charged object has gained electrons
Number of electrons > number of protons
A positively charged object has lost electrons
Number of protons > number of electrons

 In this simulation, a balloon is rubbed with a piece of wool. Observe the charges the balloon acquires and what type of charge it has.
In this simulation, a balloon is rubbed with a piece of wool. Observe the charges the balloon acquires and what type of charge it has.
What happens to the piece of wool and what type of charge does it have?
Rub another balloon with the piece of wool and bring the two balloons close to each other, then determine the type of force between them.
Bring the balloon close to the piece of wool and determine the type of force between them.
 Based on the previous experiment, answer the following questions:
Based on the previous experiment, answer the following questions:
 Click here to show solution
Click here to show solution
 Useful information: Charge of an object
Useful information: Charge of an object
The charge of an object is calculated by the relation:
q = ±n .e
\[q\] Charge of the object
\[n\] Number of electrons gained or lost
\[e=1.602× 10^{-19}c\] Elementary charge of electron
If the object gained electrons we put a negative sign, and if it lost electrons we put a positive sign
We use color codes for charges
Positive charges are red
Negative charges are blue

Example: 1

A glass rod was rubbed with a piece of wool, causing the glass rod to become positively charged. This means the rod:
 Click here to show solution
Click here to show solution
Choose the correct answer
Example: 2

A balloon was rubbed with a piece of wool and became negatively charged with a charge of:
8 × 10-19c
This means the piece of wool:
\[q_e=1.6× 10^{-19} C\]

 Click here to show solution
Click here to show solution
Choose the correct answer

Conductors, Insulators, Semiconductors and Superconductors
Conducting Materials:
Materials that can conduct electric current because they contain free electrons capable of moving through the material like copper, iron, aluminum
Insulating Materials:
Materials that cannot conduct electric current because their electrons are bound to the nucleus and cannot move freely through the material like wood, plastic, ebonite
Semiconductors: Materials that can conduct current under special conditions like germanium and silicon (Group 14 elements)
These materials contain four valence electrons that form covalent bonds between atoms at absolute zero temperature (273-)
At absolute zero they cannot conduct, but at room temperature some bonds break and they become conductive

When doping semiconductors with impurities from Group 15 elements (donors) type
\[N \]
Doping ratio is about one impurity atom per million host atoms, making electrons the charge carriers

When doping semiconductors with impurities from Group 13 elements (acceptors) type
\[p\]
Doping ratio is about one impurity atom per million host atoms, making holes the charge carriers

Superconductors:
Materials whose resistance to current flow is almost zero, and these materials only work at very low temperatures like titanium.

Types of Electric Charging
Types of Electric Charging
Charging by Friction
Scientific Explanation:
This phenomenon occurs when two different objects are rubbed together, causing electrons to transfer from one object to another due to differences in the materials' ability to lose or gain electrons.

Electromagnetic Interaction:
- Material with higher electron affinity (like wool) loses electrons
- Material with lower electron affinity (like plastic) gains electrons
Practical Applications:
- Manufacturing Van de Graaff generators
- Electrostatic painting systems
- Air purification systems for suspended particles
- Educational experiments to understand static electricity
Primary Purpose:
Generating static electric charges by transferring electrons between materials, maintaining the acquired charge for a relatively long time.
Charging by Induction
Charging by Induction
Definition of Induction Charging:
A process of separating electric charges in a conducting body without direct contact with the charged body, where the electric charges in the neutral body are affected by the electric field of the charged body.
Process Steps:
- Bring a charged object (like a negatively charged rod) close to a neutral conducting body
- Polarization occurs in the neutral body (positive charges approach, negative charges move away)
- Connect the conducting body to ground (earthing)
- Remove the ground connection then move the charged object away
- The conducting body becomes charged with opposite charge to the inducing body
Practical Example:
When bringing a negatively charged balloon close to an aluminum can:
- Positive charges in the can are attracted toward the balloon
- Negative charges move to the far side
- If the can is grounded, negative charges transfer to earth
- After removing the balloon, the can remains positively charged
Key Characteristics:
- Doesn't require direct contact between objects
- Resulting charge is opposite to the inducing charge
- Causes temporary change in charge distribution
- Based on the law of conservation of electric charge
Comparison Between Conductors and Insulators
Comparison Between Charging Conductors and Insulators
Property
Conductors
Insulators
Electron Movement
Free moving electrons
Electrons bound to their atoms
Electrical Conductivity
✅ Excellent electrical conduction
❌ No electrical conduction
Thermal Conductivity
✅ Good heat conduction
❌ Poor heat conduction
Examples
Copper, silver, aluminum
Wood, glass, rubber
Surface Charging
Charges spread on surface
Charges remain in place
Applications
Electrical wiring
Wire insulation
Scientific Explanation:
Conductors: Contain free electrons in the outer shell of their atoms, allowing easy transfer of electric charges through the material.
Insulators: Have electrons strongly bound to the atomic nucleus, preventing free movement and thus preventing electric current flow.
Charge Conservation Law:
When charging a conductor:
- Charges move to the outer surface
- Charges distribute evenly
Environmental Impact:
Insulators play a crucial role in:
- Preventing electric shocks
- Maintaining current stability
- Reducing energy loss
When bringing a charged object close to a conducting material, charge rearrangement occurs, while in an insulating material the positive charge center moves away from the negative without actual movement
Charging an Electroscope by Induction
Charging an Electroscope by Induction (Negative Charge)
Step 1: Bring the Charged Object Close
Bring a positively charged conductor (+) close to the electroscope disk.
→ Causes charge redistribution (electrostatic induction)
Qelectroscope = σ+A - σ-A
Step 2: Grounding (Touching the Base)
When touching the base, free electrons move from earth to electroscope
ΔQ = e- × n (where n is number of electrons)
→ The electroscope gets rid of positive charges
Step 3: Remove Source Then Finger
After removing ground connection and moving charged object away:
Qfinal = -|Qsource|
→ The electroscope becomes negatively charged
Practical Applications
- Charging objects in electrostatic painting systems
- Copying documents in photocopiers
- Air purification systems for suspended particles
- Charging balloons in educational science experiments
Fundamental Conservation Law
ΣQbefore = ΣQafter
(Conservation of electric charge)
This is the process of charging a conducting body without contact
Steps to charge an electroscope with negative charge by induction:
Bring a positively charged conductor close to the electroscope (causes charge redistribution in the electroscope)
Touch the electroscope base with your hand (grounding) - free charges not affected by attractive force discharge
Remove your hand then move the charged object away - the electroscope becomes negatively charged
Charging by Contact
Scientific Explanation:
The process of transferring electric charge through direct contact between a charged object and a neutral one, where charges transfer until the system reaches electrostatic equilibrium.
Transfer Mechanism:
- If the charged object is negative: excess electrons transfer to the neutral object
- If the charged object is positive: electrons are drawn from the neutral object
Practical Applications:
- Electrical grounding systems
- Rechargeable battery charging
- Wireless power transfer systems (through direct contact)
- Voltage measuring devices
Primary Purpose:
Distributing electric charges equally between contacting objects, with the ability to control the amount and type of transferred charge.
When a positively charged conductor touches another neutral conductor of the same shape, part of the charge transfers to the neutral conductor, but the charges aren't necessarily shared equally
Unless the charged object is identical to the neutral conductor, then charge is shared equally

Example: 3


One of the following conductors has increased its mass
Given:
Approach occurred in images \[A\;\;\;\;,B\] Contact occurred in images \[C\;\;\;\;,D\]

 Click here to show solution
Click here to show solution
Choose the correct answer
Example: 4


Two identical neutral conductors were brought close to a positively charged object
as shown in the figure
then grounded
and then the ground connection was removed
and the charged object was moved away
and the conductors were separated from each other
then the charges on the conductors will be:

 Click here to show solution
Click here to show solution
Choose the correct answer
Example: 5

When a positively charged conductor with charge of
6ᶙc
touches another identical neutral conductor and they are separated, the charge on each conductor will be

 Click here to show solution
Click here to show solution
Choose the correct answer
Static Electricity and Coulomb's Law

I am the scientist Coulomb "
I studied the mutual forces between electric charges and found that
$F \propto {q_1}{q_2}$
The electric force is directly proportional to the product of the two charges
$F \propto \frac{1}{{{r^2}}}$
The electric force is inversely proportional to the square of the distance between the charges
The electric force changes with the medium
So I arrived at Coulomb's constant whose value changes with the medium
$k = 9.0 \times {10^9}\frac{{{\rm{N}}{{\rm{m}}^{\rm{2}}}}}{{{{\rm{C}}^{\rm{2}}}}}$
$k=1/4\pi\epsilon_0$. Where $\epsilon_0 = 8.85\times{10^{ - 12}}\frac{{{{\rm{C}}^{\rm{2}}}}}{{{\rm{N}}{{\rm{m}}^{\rm{2}}}}}$
So I arrived at the following relationship
\[F = k\frac{{{q_1}{q_2}}}{{{r^2}}} \tag{1} \label{1}\]


In this simulation, we will verify the relationship between the force magnitude and the charge magnitudes
and verify the relationship between the force magnitude and the distance between charges by clicking on the icon on the right. The first one shows the relationship with distance between charges, and the icon below it shows the relationship with charge magnitudes. You should first fix the charge magnitude and change the distance and observe the graph, then fix the distance and change one charge magnitude (the first charge) and observe the graph. What do you conclude?

Useful information: The mutual force between two charges
There are two types of electric forces: attraction and repulsion
Attractive force occurs between opposite charges 
Repulsive force occurs between like charges 

Note that no matter what type of force is between the charges
The directions of the mutual forces between the charges are opposite
The magnitude of the force exerted by the first charge on the second equals the magnitude of the force exerted by the second charge on the first but in the opposite direction
F12 = -F21
For every action there is a reaction (Newton's third law)

Example :1
Two charges of different types where the first is twice the second \[q_1=+2q ,q_2=-q\] If an electric force from the first charge acts on the second
\[F_{12}= 10 N \] to the right
Then the force exerted by the second charge on the first equals
 Click here to show solution
Click here to show solution
Choose the correct answer
Example :2
Two charges with the same magnitude as in the figure, the distance
between them
0.2 m
If the mutual electric force
between them equals
0. 4 N
Then the magnitude of each charge equals
 Click here to show solution
Click here to show solution
Choose the correct answer


:Superposition of Electric Forces
In the figure below we have three electric charges

Determine the direction of the force acting on the first charge from the second charge and write the relationship expressing it
\[..........................................................\]
 Click here to show solution
Click here to show solution
Determine the direction of the force acting on the first charge from the third charge and write the relationship expressing it
\[..........................................................\]
 Click here to show solution
Click here to show solution
If the three charges were equal in magnitude, what is the direction of the force acting on the first charge
\[..........................................................\]
 Click here to show solution
Click here to show solution
In this simulation we place more than two charges next to each other in a straight line and determine the force acting on one charge and determine the direction of the force
Solved Example
Three point charges were placed at the vertices of a right-angled triangle as in the figure
\[q_1= 2\;µc\;\;\;\;\;\;\;q_2=-3\;µc\;\;\;\;\;\;\;q_3=4\;µc\] and the distance between the charges equals
\[r_{12}=0.3\;m\;\;\;\;\;\;\;\;\;\; r_{13}=0.4\;m\]
Calculate the electric force acting on the first charge

Solution
\[Fe_{21}=K\frac {q_1.q_2}{r_{12}^2}=9×10^9×\frac {2×10^{-6}×3×10^{-6}}{0.3^2}=0.6 N \]
\[Fe_{31}=K\frac {q_1.q_3}{r_{13}^2}=9×10^9×\frac {2×10^{-6}×4×10^{-6}}{0.2^2}=1.8 N \]
\[F_{net}=\sqrt {F_{21}^2+F_{31}^2}=\sqrt {0.6^2+1.8^2}=1.9 N \]
 Direction
\[𝜃=tan^{-1}\frac {Fe_{21}}{Fe_{31}}=tan^{-1}\frac {0.6}{1.8}=18.4^0\]
Direction
\[𝜃=tan^{-1}\frac {Fe_{21}}{Fe_{31}}=tan^{-1}\frac {0.6}{1.8}=18.4^0\]
Electric Force Between Two Charges
Coulomb's Law
The electric force between two point charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between them:
\[F=K.\frac{q_1.q_2}{r^2}\]
Where:
k = Coulomb's constant ≈ 8.9875×10⁹ N·m²/C²
Types of Electric Forces
1. Attractive Force
Occurs when the two charges are of opposite types (one positive and one negative)
Example: Attraction between electrons and nucleus in an atom
2. Repulsive Force
Occurs when the two charges are of the same type (both positive or both negative)
Example: Repulsion between two similarly charged balloons
Important Properties
- Mutual force (equal in magnitude and opposite in direction)
- Central force (acts along the line joining the charges)
- Decreases in strength with increasing distance between charges
- Depends on the insulating medium between the charges
Practical Applications
- Design of electronic circuits
- Operation of capacitors
- Lightning phenomenon
- Laser printers
Important Notes:
1. The force is repulsive if the charges are similar
2. The force is attractive if the charges are different
3. Unit of force: Newton (N)
4. Mutual interaction according to Newton's third law
Electric Force Between Three Charges
Electric Force Between Three Charges in a Straight Line
Coulomb's Law:
Force between two charges:
\[F=K.\frac{q_1.q_2}{r^2}\]
Resultant Force Determination:
- Determine the direction of force between each pair (repulsion ←← or attraction →←)
- Calculate the force magnitude between each pair using the law
- Add the forces vectorially according to their direction
Direction Determination Table (for middle charge):
Charge Arrangement
Force Direction
Example
(+ , + , +)
← if closer to left, → if closer to right
Q1=+2C, Q2=+3C, Q3=+5C
(- , - , -)
→ if closer to left, ← if closer to right
Q1=-4C, Q2=-1C, Q3=-3C
(+ , + , -)
← from left (repulsion), → from right (attraction)
Q1=+5C, Q2=+2C, Q3=-6C
(- , + , +)
→ from left (attraction), ← from right (repulsion)
Q1=-3C, Q2=+4C, Q3=+1C
Notes:
- Charge sign determines force type (repulsion/attraction)
- Distance between charges determines force strength
- Resultant force direction is determined by comparing force magnitudes
Electric Force in Right Triangle
Analysis of Electric Forces in Right Triangle
Geometric Configuration:
Three point charges (q₁, q₂, q₃) placed at:
- q₁ and q₂ at the short sides
- q₃ at the right angle vertex (90° angle)
Basic Coulomb's Law:
\[F=K.\frac{q_1.q_2}{r^2}\]
where kₑ = 8.99×10⁹ N·m²/C²
Steps to Calculate Resultant on q₃:
- Calculate force between q₁ and q₃ \[F_{12}=K.\frac{q_1.q_2}{a^2}\]
- Calculate force between q₂ and q₃: \[F_{23}=K.\frac{q_2.q_3}{b^2}\]
- Resolve forces into components:
- F₁₃ → horizontal component (F₁₃x) and vertical component (F₁₃y)
- F₂₃ → horizontal component (F₂₃x) and vertical component (F₂₃y)
- Total resultant:
\[Fₜₒₜₐₗ_x = ΣFₓ\]
\[Fₜₒₜₐₗ_y = ΣFᵧ\]
Determining Resultant Direction:
\[ θ = tan^{-1}\frac {(Fₜₒₜₐₗ_y )}{ (Fₜₒₜₐₗ_x)}\]
Important Notes:
- Consider charge signs (attraction/repulsion)
- Distances calculated using Pythagorean theorem when needed
- Direction depends on charge nature:
Charge Types
Force Direction
Same
Repulsion
Different
Attraction
Coulomb's Law Simulation
Force Between Two Charges
Three Charges in Straight Line
Right Triangle

:Applications of Static Electricity
Although static electricity is often considered a nuisance,
it has many practical applications in various industries.
These applications utilize the principles of static electricity to achieve useful results.
Electrostatic Precipitators

Electrostatic precipitators are used to remove particles from industrial emissions.
By applying a high voltage charge to particles in the exhaust stream, these devices cause the particles to become charged and then adhere to oppositely charged plates,
effectively removing them from the air.
Copiers and Laser Printers

Copiers and laser printers rely on static electricity to transfer fine toner powder to paper.
The drum or belt inside the machine is charged with static electricity, attracting toner particles to form an image,
which is then transferred to paper and fused using heat
Paint Spraying

Electrostatic spray painting uses static electricity to improve paint adhesion and reduce overspray.
By charging paint particles and the object to be painted with opposite charges,
the paint is attracted to the object, resulting in more even and efficient painting.


"Gravitational force between two bodies
"\[F = G\frac{{{m_1}{m_2}}}{{{r^2}}} \]
and the electric force between two charges
\[F = k\frac{{{q_1}{q_2}}}{{{r^2}}} \]
Question: What are the similarities and differences between the two relationships without the results in the table
Electric Force
Gravitational Force
\[\;\;\;\;\;\;\;\;\;\;\]
\[1 - ................\]
\[2 - .................\]
\[1 - ................\]
\[2 - .................\]
Similarities
\[1 - ................\]
\[2 - .................\]
\[1 - ................\]
\[2 - .................\]
Differences


المصدر
https://phet.colorado.edu/sims/html/balloons-and-static-electricity/latest/balloons-and-static-electricity_ar_SA.html
🧮 Calculator
🗑️
✏️ قلم
Static Electricity |


Negative (-) and Positive (+)
What does it mean for an object to be positively charged?
What does it mean for an object to be negatively charged?

Any material consists of
A nucleus containing positively charged protons and neutral neutrons
Electrons with negative charge orbit around the nucleus
Is the atom electrically neutral?
Because the number of protons = number of electrons
Proton charge = Electron charge
\[ 𝑞_𝑒 = 𝑞_𝑝 = 1.602 X 10^{-19}C\]
Electrons are the particles that are gained or lost
Charged objects contain protons and electrons but their numbers differ
A negatively charged object has gained electrons
Number of electrons > number of protons
A positively charged object has lost electrons
Number of protons > number of electrons
The charge of an object is calculated by the relation:
q = ±n .e
\[q\] Charge of the object
\[n\] Number of electrons gained or lost
\[e=1.602× 10^{-19}c\] Elementary charge of electron
If the object gained electrons we put a negative sign, and if it lost electrons we put a positive sign
We use color codes for charges
Positive charges are red
Negative charges are blue
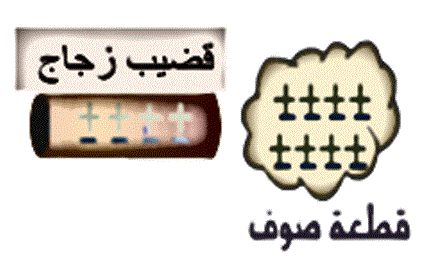
A glass rod was rubbed with a piece of wool, causing the glass rod to become positively charged. This means the rod:
Choose the correct answer A balloon was rubbed with a piece of wool and became negatively charged with a charge of:
Choose the correct answer These materials contain four valence electrons that form covalent bonds between atoms at absolute zero temperature (273-)
At absolute zero they cannot conduct, but at room temperature some bonds break and they become conductive
When doping semiconductors with impurities from Group 15 elements (donors) type
\[N \] Doping ratio is about one impurity atom per million host atoms, making electrons the charge carriers
When doping semiconductors with impurities from Group 13 elements (acceptors) type
\[p\]
Doping ratio is about one impurity atom per million host atoms, making holes the charge carriers
Scientific Explanation:
Electromagnetic Interaction:
Generating static electric charges by transferring electrons between materials, maintaining the acquired charge for a relatively long time.
A process of separating electric charges in a conducting body without direct contact with the charged body, where the electric charges in the neutral body are affected by the electric field of the charged body. When bringing a negatively charged balloon close to an aluminum can: Conductors: Contain free electrons in the outer shell of their atoms, allowing easy transfer of electric charges through the material. Insulators: Have electrons strongly bound to the atomic nucleus, preventing free movement and thus preventing electric current flow. When charging a conductor:
Insulators play a crucial role in:
Bring a positively charged conductor (+) close to the electroscope disk.
When touching the base, free electrons move from earth to electroscope
After removing ground connection and moving charged object away:
ΣQbefore = ΣQafter
Scientific Explanation:
Transfer Mechanism:
Distributing electric charges equally between contacting objects, with the ability to control the amount and type of transferred charge.
One of the following conductors has increased its mass
Choose the correct answer Two identical neutral conductors were brought close to a positively charged object Choose the correct answer When a positively charged conductor with charge of Choose the correct answer $F \propto {q_1}{q_2}$ The electric force is directly proportional to the product of the two charges
$F \propto \frac{1}{{{r^2}}}$ The electric force is inversely proportional to the square of the distance between the charges
So I arrived at the following relationship There are two types of electric forces: attraction and repulsion
Attractive force occurs between opposite charges Repulsive force occurs between like charges Note that no matter what type of force is between the charges
The directions of the mutual forces between the charges are opposite
The magnitude of the force exerted by the first charge on the second equals the magnitude of the force exerted by the second charge on the first but in the opposite direction
F12 = -F21
For every action there is a reaction (Newton's third law)
Two charges of different types where the first is twice the second \[q_1=+2q ,q_2=-q\] If an electric force from the first charge acts on the second
\[F_{12}= 10 N \] to the right
Then the force exerted by the second charge on the first equals
Choose the correct answer Two charges with the same magnitude as in the figure, the distance
between them
Choose the correct answer In the figure below we have three electric charges
Determine the direction of the force acting on the first charge from the second charge and write the relationship expressing it
Determine the direction of the force acting on the first charge from the third charge and write the relationship expressing it
\[..........................................................\]
If the three charges were equal in magnitude, what is the direction of the force acting on the first charge
\[..........................................................\]
The electric force between two point charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between them: \[F=K.\frac{q_1.q_2}{r^2}\] Where: Occurs when the two charges are of opposite types (one positive and one negative) Example: Attraction between electrons and nucleus in an atom Occurs when the two charges are of the same type (both positive or both negative) Example: Repulsion between two similarly charged balloons 1. The force is repulsive if the charges are similar Force between two charges:
\[F=K.\frac{q_1.q_2}{r^2}\]
Three point charges (q₁, q₂, q₃) placed at:
\[ θ = tan^{-1}\frac {(Fₜₒₜₐₗ_y )}{ (Fₜₒₜₐₗ_x)}\] "Gravitational force between two bodies "\[F = G\frac{{{m_1}{m_2}}}{{{r^2}}} \] 
In this simulation, a balloon is rubbed with a piece of wool. Observe the charges the balloon acquires and what type of charge it has.
What happens to the piece of wool and what type of charge does it have?
Rub another balloon with the piece of wool and bring the two balloons close to each other, then determine the type of force between them.
Bring the balloon close to the piece of wool and determine the type of force between them.
Based on the previous experiment, answer the following questions:
 Click here to show solution
Click here to show solution
Useful information: Charge of an object




8 × 10-19c
This means the piece of wool:
\[q_e=1.6× 10^{-19} C\]

Conductors, Insulators, Semiconductors and Superconductors
Insulating Materials:
Materials that cannot conduct electric current because their electrons are bound to the nucleus and cannot move freely through the material like wood, plastic, ebonite
Semiconductors: Materials that can conduct current under special conditions like germanium and silicon (Group 14 elements)


Superconductors:
Materials whose resistance to current flow is almost zero, and these materials only work at very low temperatures like titanium.
Types of Electric Charging
Charging by Friction
This phenomenon occurs when two different objects are rubbed together, causing electrons to transfer from one object to another due to differences in the materials' ability to lose or gain electrons.
- Material with higher electron affinity (like wool) loses electrons
- Material with lower electron affinity (like plastic) gains electrons
Practical Applications:
Primary Purpose:
Charging by Induction
Definition of Induction Charging:
Process Steps:
Practical Example:
Key Characteristics:
Comparison Between Charging Conductors and Insulators
Property
Conductors
Insulators
Electron Movement
Free moving electrons
Electrons bound to their atoms
Electrical Conductivity
✅ Excellent electrical conduction
❌ No electrical conduction
Thermal Conductivity
✅ Good heat conduction
❌ Poor heat conduction
Examples
Copper, silver, aluminum
Wood, glass, rubber
Surface Charging
Charges spread on surface
Charges remain in place
Applications
Electrical wiring
Wire insulation
Scientific Explanation:
Charge Conservation Law:
- Charges move to the outer surface
- Charges distribute evenlyEnvironmental Impact:
- Preventing electric shocks
- Maintaining current stability
- Reducing energy loss
When bringing a charged object close to a conducting material, charge rearrangement occurs, while in an insulating material the positive charge center moves away from the negative without actual movement
Charging an Electroscope by Induction (Negative Charge)
Step 1: Bring the Charged Object Close
→ Causes charge redistribution (electrostatic induction)
Qelectroscope = σ+A - σ-A
Step 2: Grounding (Touching the Base)
ΔQ = e- × n (where n is number of electrons)
→ The electroscope gets rid of positive charges
Step 3: Remove Source Then Finger
Qfinal = -|Qsource|
→ The electroscope becomes negatively charged
Practical Applications
Fundamental Conservation Law
(Conservation of electric charge)
This is the process of charging a conducting body without contact
Steps to charge an electroscope with negative charge by induction:
Bring a positively charged conductor close to the electroscope (causes charge redistribution in the electroscope)
Touch the electroscope base with your hand (grounding) - free charges not affected by attractive force discharge
Remove your hand then move the charged object away - the electroscope becomes negatively charged
Charging by Contact
The process of transferring electric charge through direct contact between a charged object and a neutral one, where charges transfer until the system reaches electrostatic equilibrium.
- If the charged object is negative: excess electrons transfer to the neutral object
- If the charged object is positive: electrons are drawn from the neutral object
Practical Applications:
Primary Purpose:
Unless the charged object is identical to the neutral conductor, then charge is shared equally


Given:
Approach occurred in images \[A\;\;\;\;,B\] Contact occurred in images \[C\;\;\;\;,D\]




as shown in the figure
then grounded
and then the ground connection was removed
and the charged object was moved away
and the conductors were separated from each other
then the charges on the conductors will be:

 Click here to show solution
Click here to show solution

6ᶙc
touches another identical neutral conductor and they are separated, the charge on each conductor will be

Static Electricity and Coulomb's Law

I am the scientist Coulomb "
I studied the mutual forces between electric charges and found that
The electric force changes with the medium
So I arrived at Coulomb's constant whose value changes with the medium
$k = 9.0 \times {10^9}\frac{{{\rm{N}}{{\rm{m}}^{\rm{2}}}}}{{{{\rm{C}}^{\rm{2}}}}}$
$k=1/4\pi\epsilon_0$. Where $\epsilon_0 = 8.85\times{10^{ - 12}}\frac{{{{\rm{C}}^{\rm{2}}}}}{{{\rm{N}}{{\rm{m}}^{\rm{2}}}}}$
\[F = k\frac{{{q_1}{q_2}}}{{{r^2}}} \tag{1} \label{1}\]

Useful information: The mutual force between two charges


0.2 m
If the mutual electric force
between them equals
0. 4 N
Then the magnitude of each charge equals

:Superposition of Electric Forces

\[..........................................................\]



Solved Example
Three point charges were placed at the vertices of a right-angled triangle as in the figure
\[q_1= 2\;µc\;\;\;\;\;\;\;q_2=-3\;µc\;\;\;\;\;\;\;q_3=4\;µc\] and the distance between the charges equals
\[r_{12}=0.3\;m\;\;\;\;\;\;\;\;\;\; r_{13}=0.4\;m\]
Calculate the electric force acting on the first charge

Solution
\[Fe_{21}=K\frac {q_1.q_2}{r_{12}^2}=9×10^9×\frac {2×10^{-6}×3×10^{-6}}{0.3^2}=0.6 N \]
\[Fe_{31}=K\frac {q_1.q_3}{r_{13}^2}=9×10^9×\frac {2×10^{-6}×4×10^{-6}}{0.2^2}=1.8 N \]
\[F_{net}=\sqrt {F_{21}^2+F_{31}^2}=\sqrt {0.6^2+1.8^2}=1.9 N \]

Electric Force Between Two Charges
Coulomb's Law
k = Coulomb's constant ≈ 8.9875×10⁹ N·m²/C²Types of Electric Forces
1. Attractive Force
2. Repulsive Force
Important Properties
Practical Applications
Important Notes:
2. The force is attractive if the charges are different
3. Unit of force: Newton (N)
4. Mutual interaction according to Newton's third law
Electric Force Between Three Charges in a Straight Line
Coulomb's Law:
Resultant Force Determination:
Direction Determination Table (for middle charge):
Charge Arrangement
Force Direction
Example
(+ , + , +)
← if closer to left, → if closer to right
Q1=+2C, Q2=+3C, Q3=+5C
(- , - , -)
→ if closer to left, ← if closer to right
Q1=-4C, Q2=-1C, Q3=-3C
(+ , + , -)
← from left (repulsion), → from right (attraction)
Q1=+5C, Q2=+2C, Q3=-6C
(- , + , +)
→ from left (attraction), ← from right (repulsion)
Q1=-3C, Q2=+4C, Q3=+1C
Notes:
Analysis of Electric Forces in Right Triangle
Geometric Configuration:
- q₁ and q₂ at the short sides
- q₃ at the right angle vertex (90° angle)
Basic Coulomb's Law:
\[F=K.\frac{q_1.q_2}{r^2}\]
where kₑ = 8.99×10⁹ N·m²/C²
Steps to Calculate Resultant on q₃:
\[Fₜₒₜₐₗ_x = ΣFₓ\]
\[Fₜₒₜₐₗ_y = ΣFᵧ\]
Determining Resultant Direction:
Important Notes:
Charge Types
Force Direction
Same
Repulsion
Different
Attraction
Force Between Two Charges
Three Charges in Straight Line
Right Triangle
:Applications of Static Electricity
Although static electricity is often considered a nuisance,
it has many practical applications in various industries.
These applications utilize the principles of static electricity to achieve useful results.
Electrostatic Precipitators

Electrostatic precipitators are used to remove particles from industrial emissions.
By applying a high voltage charge to particles in the exhaust stream, these devices cause the particles to become charged and then adhere to oppositely charged plates,
effectively removing them from the air.
Copiers and Laser Printers

Copiers and laser printers rely on static electricity to transfer fine toner powder to paper.
The drum or belt inside the machine is charged with static electricity, attracting toner particles to form an image,
which is then transferred to paper and fused using heat
Paint Spraying

Electrostatic spray painting uses static electricity to improve paint adhesion and reduce overspray.
By charging paint particles and the object to be painted with opposite charges,
the paint is attracted to the object, resulting in more even and efficient painting.

and the electric force between two charges
\[F = k\frac{{{q_1}{q_2}}}{{{r^2}}} \]
Electric Force
Gravitational Force
\[\;\;\;\;\;\;\;\;\;\;\]
\[1 - ................\]
\[2 - .................\]
\[1 - ................\]
\[2 - .................\]
Similarities
\[1 - ................\]
\[2 - .................\]
\[1 - ................\]
\[2 - .................\]
Differences

المصدر
https://phet.colorado.edu/sims/html/balloons-and-static-electricity/latest/balloons-and-static-electricity_ar_SA.html
 Physics
Physics



No comments:
Post a Comment